Explore the Mystery Of Sodium Ion Batteries: Taking 12V Sodium Ion Battery And 48V Sodium ion Battery As Examples
the working principle of sodium ion battery
The battery industry is experiencing a surge in innovation, driven by the pursuit of more efficient and higher-performing technologies that can offer significant market advantages. Recently, there has been a resurgence of interest in sodium ion battery due to their economic benefits. Sodium-ion batteries are gaining traction in energy storage and electric mobility, but several limitations still need to be addressed before they can be widely commercialized. Let's explore the characteristics and potential of sodium batteries. This article will take 12v sodium ion battery and 48v sodium ion battery as an example to explore the mystery of sodium-ion batteries.
What Are Sodium Batteries and How Do They Work?
Sodium-ion batteries operate on principles similar to lithium-ion batteries. Both sodium and lithium are alkali metals located in Group 1 of the periodic table, sharing many physical and chemical properties. Research on sodium batteries began between 1970 and 1990, around the same time as lithium batteries. However, lithium batteries were commercialized more successfully, sidelining sodium batteries.
Similarities with Lithium Batteries
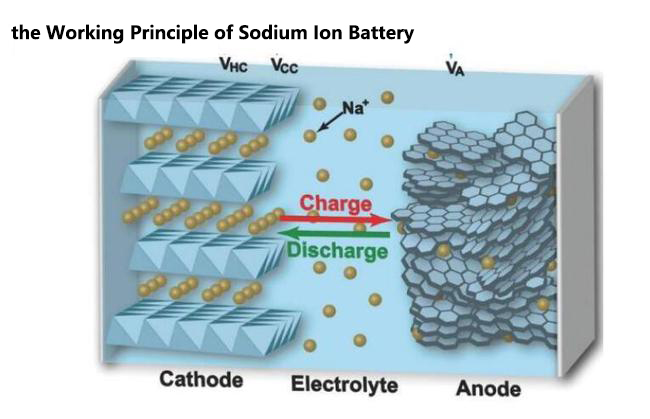
Sodium-ion and lithium-ion batteries share the same basic working principle. Both use ions to store and transfer energy. During charging, sodium ions move from the cathode (positive electrode) to the anode (negative electrode) through the electrolyte. During discharge, the ions return to the cathode, generating an electric current.
A sodium battery consists of a cathode made of sodium-containing material, a carbon-based anode, and a liquid electrolyte with sodium ions. This setup allows ions to move between the cathode and anode, enabling energy storage and release.
Differences from Lithium Batteries
Chemically, sodium differs significantly from lithium. Sodium ions are larger and heavier, leading to greater mechanical stress during charge cycles, which causes faster cell degradation. Consequently, sodium batteries have a shorter cycle life compared to lithium batteries. Additionally, sodium batteries have a lower standard reduction potential, resulting in a lower maximum voltage and energy density. A sodium battery stores about 40% less energy than a lithium battery of the same weight.
Pros and Cons of Sodium Batteries
Benefits
Abundance and Low Cost: Sodium is the sixth most abundant element in the Earth's crust, making it economically competitive.
Safety: Sodium-based cells are non-flammable and less prone to explosions or short circuits.
Temperature Resilience: They operate effectively in a wider temperature range (-20°C to 60°C) compared to lithium batteries (0°C to 50°C).
Environmental Impact: The extraction and processing of sodium require less energy, reducing environmental impact.
Limitations
Low Energy Density: Sodium batteries have lower energy density (140-160 Wh/kg) compared to lithium-ion batteries (180-250 Wh/kg).
Short Cycle Life: The larger mass of sodium ions causes greater mechanical stress, leading to faster degradation of the anode material, typically graphite.
Taking 12V Sodium Ion Battery and 48V Sodium Ion Battery as examples, the application of sodium-ion batteries was discussed
12V sodium ion battery is commonly used in small-scale energy storage devices, such as home photovoltaic storage systems, emergency power supplies, smart home systems, and more. These systems have certain requirements for battery weight, size, cost, and cycle life. Due to the high cost-performance ratio and long service life of sodium ion batteries, 12V sodium ion batteries perform exceptionally well in these applications. Their lower cost makes them more accessible for household users, especially in areas where stable long-term power supply is needed. The advantages of sodium ion batteries become even more prominent in such regions.
48V sodium ion battery is typically used in larger-scale energy storage applications, such as electric vehicles (EVs), photovoltaic power storage, wind energy storage systems, and more. In these systems, 48V battery packs can provide higher voltage and larger energy output to meet the demand for greater power.
For example, in the electric vehicle sector, 48V sodium ion battery is emerging as a lightweight and cost-effective alternative, which could become a trend in the future. Sodium ion batteries have made improvements in high-power output and fast charging, enabling electric vehicles to have longer range and shorter charging times. Additionally, their lower cost also effectively reduces the overall cost of electric vehicles.
In photovoltaic and wind energy storage systems, 48V sodium ion battery also holds significant potential. By storing electricity generated from solar or wind power, these batteries help balance the instability of energy supply and improve the efficiency of energy utilization. The high capacity and stability of 48V sodium ion batteries have made them increasingly widely used in these large-scale energy storage systems.
The Future of Sodium-Ion Technology
The sodium ion battery market is projected to grow at an annual rate of 27% over the next decade, with production expected to increase from 10 GWh in 2025 to approximately 70 GWh by 2033. The similarity in production technology between sodium and lithium cells could facilitate the transition and make sodium-ion batteries more cost-effective.
Major players in the battery manufacturing industry, like CATL, are exploring sodium-ion technology. CATL has proposed a hybrid battery pack combining sodium-ion and lithium-ion batteries to leverage the strengths of both technologies. This innovation aims to overcome sodium batteries' drawbacks and reduce the overall cost of lithium batteries.
While sodium-ion technology faces challenges, ongoing research and significant investments are likely to overcome these barriers. Sodium ion battery could offer tangible benefits in applications where energy density is less critical, providing a viable alternative to lithium batteries.
Where can I buy a sodium ion battery?
The performance of sodium-ion batteries and other factors determine that when you choose sodium-ion batteries, you must consider all aspects of the battery supplier. As a leading battery supplier, we have more than ten years of rich experience in the lithium battery industry, and have opened more than 6 store platforms for several consecutive years, energyx is one of them, you can trust us to buy high-quality lithium batteries, including sodium ion batteries!