Graphene: the key to promoting the innovation of negative electrode materials for lithium-ion batteries in new energy vehicles
The new energy vehicle lithium-ion battery is a rechargeable secondary battery that uses lithium ions to store and release energy by moving back and forth between the positive and negative electrodes. Lithium-ion batteries have the characteristics of high specific energy, high operating voltage, long cycle life and small size, and have been widely used in electronic information products, electric vehicles, smart grids and other fields. However, the development of new energy vehicles is rapid, and its performance requirements for lithium-ion batteries are increasingly high, especially the energy saving and environmental protection of negative electrode materials, high capacity, strong stability and low cost. At present, the commercial lithium ion battery anode materials mainly include graphite, lithium titanate and silicon carbon composite materials, among which graphite is a commonly used one, but its theoretical specific capacity is only 372mA·h/g, which is difficult to meet the high energy density needs of new energy vehicles. As a battery material, graphene has a good application prospect.
Liquid phase stripping is a method for preparing single layers of graphene directly from graphite, using different liquid solvents and physical means to disperse and peel the graphite layers. The preparation process of this method is simple and does not require oxidation intercalation, and has the characteristics of energy saving and environmental protection. In order to use this method to prepare graphene at scale, it is necessary to select some specific reagents and instruments, such as N-methyl pyrrolidone, polyethylene pyrrolidone, flake graphite, diaphragm, copper foil, as well as magnetic stirrers, high-speed frozen centrifuges, high-speed shear grinding dispersion machines, atomic force microscopy, X-ray diffractometer, ultra-pure water systems, transmission electron microscopy, etc. There are 4 specific steps to prepare graphene by liquid phase stripping method.
1. the graphite is treated with N-methylpyrrolidone and butylamine by two-step non-oxidation intercalation, so that the organic molecules enter the graphite layers, reduce the van der Waals forces between the graphite layers, and obtain the treated graphite raw materials. This step can improve the strippability and dispersibility of the graphite, reducing the energy required for subsequent stripping.
2. the treated graphite raw material is mechanically stripped with a high-speed shear grinding and dispersing machine, so that the graphene sheet is stripped from the graphite surface and the graphene emulsion is formed in N-methylpyrrolidone solution. This step stabilizes the graphene sheets using the high dielectric constant and low surface tension of the N-methylpyrrolidone solution, preventing them from reaggregating.
3. the solvent replacement and re-stripping of the graphene emulsion with a surfactant aqueous solvent with a 1% concentration of polyvinylpyrrolidone were performed to obtain a more stable graphene aqueous solution. In this step, the graphene sheet can be coated with polyethylpyrrolidone as a surfactant, improving its hydrophilicity and dispersibility in water, while the sheet thickness can be further reduced by mechanical stripping again.
4. prepare graphene powder by high-speed centrifugation and freeze-drying, which is used as the raw material for the negative electrode materials of lithium-ion batteries for new energy vehicles. This step can use high-speed centrifugation method to separate fewer and multi-layer sheets, and use freeze-drying method to remove residual solvents and surfactants, and obtain high purity, large specific surface area, good electrical conductivity of graphene powder. This powder can be used as a negative electrode material for lithium-ion batteries to improve the capacity and safety of the battery and extend the cycle life of the battery. In addition, graphene powder can also be used in other fields, such as catalysis, sensing, composite materials, etc.
The negative electrode material is an important part of the new energy vehicle lithium-ion battery, which directly affects the capacity, power, safety and stability of the battery. In recent years, researchers have developed many new high-capacity anode materials, such as lithium metal, alloy materials, silicon-based materials, tin-based materials, lithium titanate and transition metal oxides. These materials have theoretical specific capacities of up to 1000mA·h/g or more, but they also face many challenges, including three main aspects.
1. The formation and inhibition of lithium dendrites. Lithium dendrites refer to the microstructure of lithium metal formed by the non-uniform deposition of lithium ions on the negative electrode surface during the charging process. Lithium dendrites can lead to effective lithium loss, increased internal resistance, capacity attenuation, SEI film destruction, diaphragm puncture, internal short circuit and other problems, which seriously affect the performance and safety of the battery.
2. Mitigation and adaptation of volume change. High capacity anode materials will undergo a large volume change in the process of lithium removal, for example, the volume change of silicon-based materials can reach 300%, and the volume change of tin-based materials can reach 260%. This drastic volume change will cause the negative material to crush, fall off, crack and other phenomena, destroy the structural integrity and electronic conductivity of the negative electrode, but also damage the stability of the SEI film, accelerate the aging and decline of the battery.
3. The improvement and coordination of electron and ion transport performance. High-capacity anode materials typically have poor electron and ion transport performance, which leads to polarization loss during charge transfer, reducing the battery's power density and cycle efficiency. In addition, the mismatch or incoordination in the electron and ion transport process can also lead to uneven distribution and deposition of lithium ions on the negative surface, exacerbating problems such as lithium dendrites and volume changes.
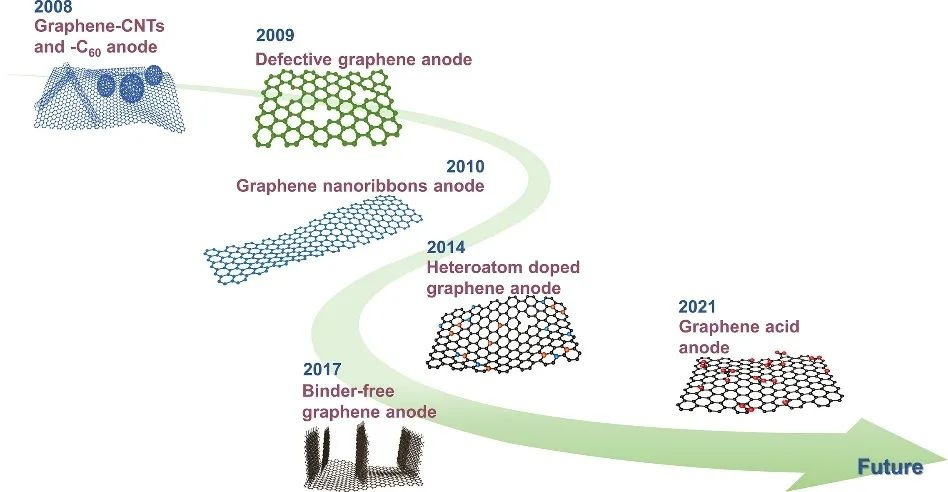
Graphene is a kind of two-dimensional nanomaterial which consists of a single layer of carbon atoms arranged in sp2 hybrid orbitals into a honeycomb structure. It has excellent mechanical, optical and chemical properties, and is considered as a new nanomaterial with good application prospects. In terms of anode materials for lithium-ion batteries, graphene has four application advantages.

